
October 14
Sono aperte le iscrizioni per il più grande evento dell'anno di Miro: Canvas 25! Iscriviti subito per assicurarti un posto per il 14 ottobre.
REGISTRATI ORA
NYC or Virtual
Sono aperte le iscrizioni per il più grande evento dell'anno di Miro: Canvas 25! Iscriviti subito per assicurarti un posto per il 14 ottobre.
REGISTRATI ORA
Sono aperte le iscrizioni per il più grande evento dell'anno di Miro: Canvas 25! Iscriviti subito per assicurarti un posto per il 14 ottobre.
REGISTRATI ORA
Sono aperte le iscrizioni per il più grande evento dell'anno di Miro: Canvas 25! Iscriviti subito per assicurarti un posto per il 14 ottobre.
REGISTRATI ORA
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori

Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.


Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.
Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.
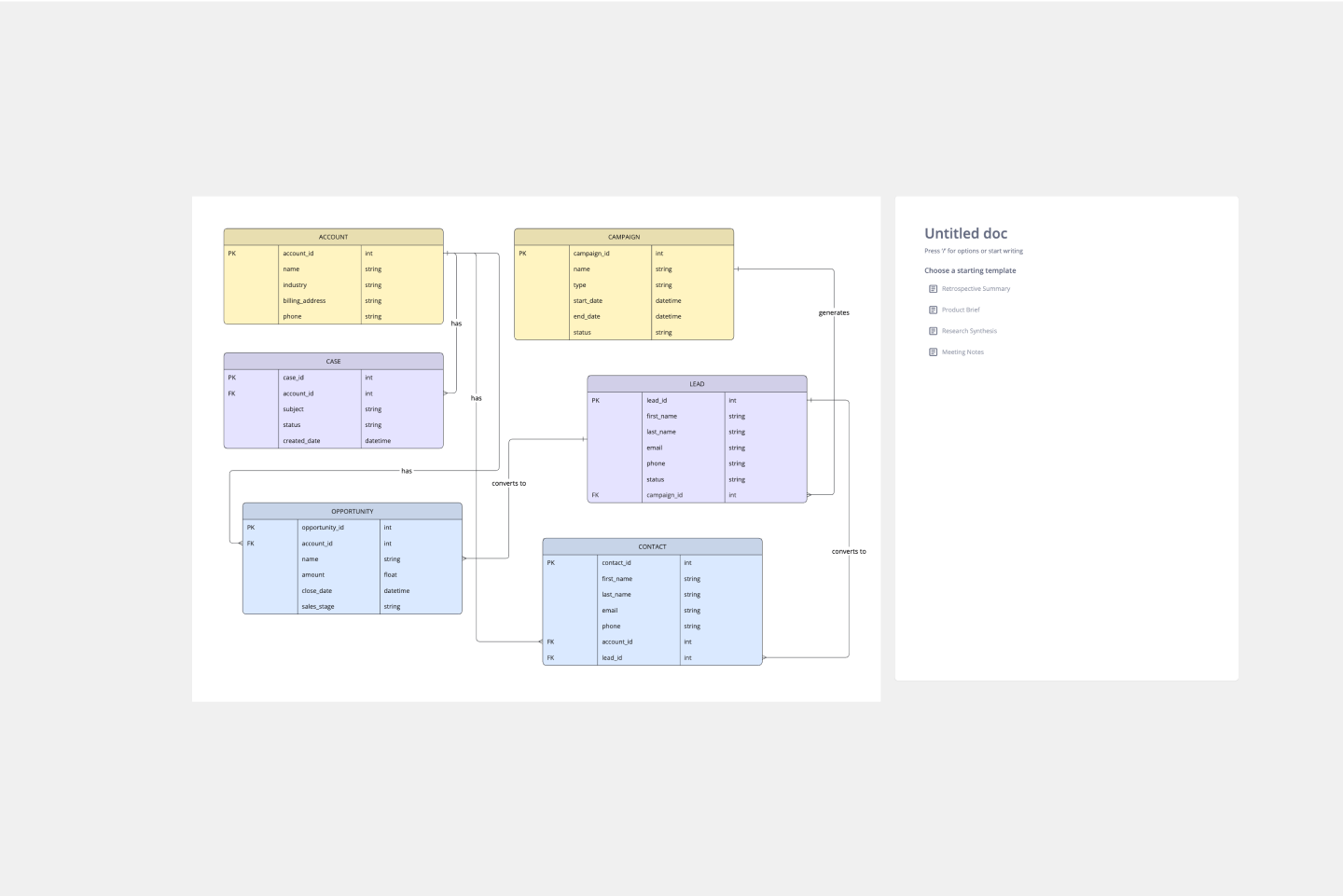
Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e
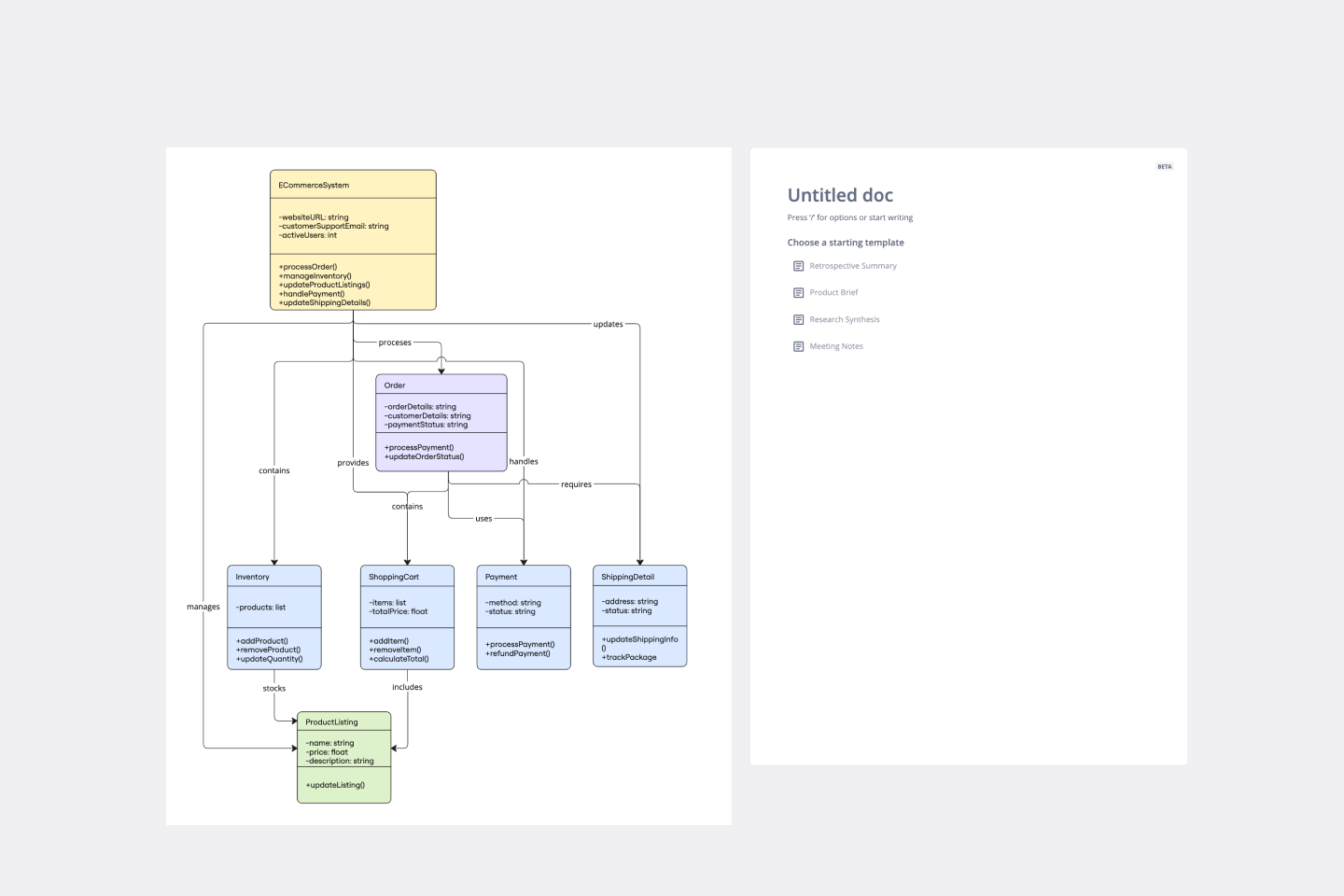
Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori

Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.


Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.
Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.

Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e

Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori

Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.


Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.
Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.

Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e

Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori
Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.

Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.

Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.

Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e

Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori
Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.

Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.

Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.

Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e

Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Visualizza diagrammi, processi e sistemi complessi più velocemente che mai
Con la creazione di diagrammi basata sull'IA puoi passare dal brainstorming alla mappa di processo, dal documento tecnico alla creazione del progetto del sistema in pochi secondi.

Oltre 90 milioni di utenti e 250.000 aziende collaborano nello spazio di lavoro per l’innovazione
Ecco come i team realizzano il prossimo grande successo
Oltre 3000 forme professionali per diagrammi
Risparmia tempo prezioso, usa l’IA per generare diagrammi
Meno fatica, diagrammi migliori

Perché i migliori team scelgono Miro per la creazione di diagrammi
Trasformazione IA
Scopri come l'IA si integra nei tuoi flussi di lavoro attuali realizzandone una mappatura su Miro. Usa il pacchetto forme IA per trascinare e rilasciare le icone IA, definire punti di contatto umani e impostare trigger di automazione.


Mappatura dei processi
Il modo più veloce per mettere in contatto i team, ottimizzare i processi e ampliare la tua attività in un unico spazio di lavoro facile da usare.
Creazione di diagrammi tecnici
Risparmia ore di progettazione manuale del sistema e concentrati su ciò che conta, mantenendo i team allineati su argomenti complessi.

Creazione di diagrammi per l'infrastruttura cloud
Migliora le prestazioni, inizia a ottimizzare i costi del cloud e visualizza la tua architettura, tutto in un unico spazio.

Progettazione organizzativa
Lo strumento più intuitivo per eseguire una mappatura della tua organizzazione e vedere il quadro generale, senza sforzo.

Wireframing
Progetta e itera le interfacce utente in modo collaborativo.

Guarda i diagrammi di Miro in azione
Scopri come i team possono creare, personalizzare e condividere rapidamente diagrammi per essere sempre allineati e agevolare la collaborazione, tutto in un unico spazio di lavoro intuitivo.

Prova i modelli più popolari su misura per il tuo team
Non serve ripartire ogni volta da zero. Esplora l'enorme libreria di modelli personalizzabili di Miro, pensati per le tue attività quotidiane.

Create a clear visual representation of a company's CRM system.
3f38d20a-0abf-4326-8f74-517a432aefc5

Visualizza il flusso dei dati e i modelli di processo con un diagramma di flusso dati.
f2ef89ce-36b3-4f49-ac7c-4a0851041eb8

Crea una guida visiva alla struttura della tua organizzazione.
c8f2189a-946b-40a3-9eff-e2954a3e3920

Il Diagramma dell'architettura AWS è una rappresentazione grafica del framework AWS, inoltre traduce le migliori pratiche quando si utilizza l'architettura Amazon Web Services
8cfe871d-e74a-41cd-b71d-908636d2507e

Deliver a clear visualization of the class structure of an e-commerce system.
5b0855eb-0609-4c1f-8d69-673cf5521888

Chiarisci i ruoli e sostituisci i processi scritti lunghi con riferimenti visivi.
0d701836-612a-46c0-8305-5a55ace59a47
Hai bisogno di aiuto per iniziare?
Accedi a corsi gratuiti per imparare a usare la tela in un batter d'occhio, esplora il nostro blog, ottieni risposte rapide dal Centro assistenza e altro ancora.
Prodotto
Soluzioni
Risorse
Azienda
Piani e prezzi
Prodotto
Soluzioni
Risorse
Azienda
Piani e prezzi
Prodotto
Soluzioni
Risorse
Azienda
Piani e prezzi
Prodotto
Soluzioni
Risorse
Azienda
Piani e prezzi





